Quality B cleanrooms are utilized for aseptic preparing, filling, and compounding procedures. They may be comparable to ISO Class five cleanrooms at relaxation and ISO Class seven cleanrooms in Procedure.
Air would be the transport technique that contaminates use to pollute an atmosphere. The ventilation program makes sure that the adverse particulates while in the air are taken off to keep up cleanroom specifications.
Smooth Walls – Softwall cleanrooms have partitions manufactured from vinyl sheets with a variety of types of finishes. They can certainly satisfy the benchmarks for just a class one hundred cleanroom.
The checking on the Quality C area should be carried out in step with top quality risk administration rules, the systematic approach for examining, managing, speaking, and reviewing challenges to the standard of a medicinal product or service during its lifespan.
See Extra › What is Quality A area in pharmaceutical business? › A cleanroom or clear area is really an atmosphere, commonly used in manufacturing or scientific investigation that has a low level of environmental pollutants such as dust, airborne microbes, aerosol particles and chemical vapors.
He has wealthy knowledge and supplies worthwhile insights and details through his articles and information on Pharmaguddu.com. For further more inquiries or collaborations, you should don’t be reluctant to reach out by means of electronic mail at Get hold of@pharmaguddu.com.
Some cleanroom HVAC systems Command the humidity to this sort of low ranges that excess machines like air ionizers are required to stop electrostatic discharge challenges. That is a specific problem throughout the semiconductor small business, since static discharge can easily problems modern-day circuit models. Alternatively, active ions during the air can damage uncovered components at the same time.
Nonetheless, compared with oral-reliable dosage sorts, parenteral formulations call for incredibly specific and essential criteria, and lift sure problems and restrictions, for instance drug steadiness problems. Primarily, alternatives, suspensions, or emulsions which have been designed for administration by injection or implantation are directly entered into a human’s systemic circulation system and so needs to be sterile and Protected for use.
As you are able class 100 area is referred to aseptic area to see, there’s lots to find out about freight class, but if you do have thoughts, FreightPros powered by NTG will be able to help you locate the correct class for the shipment.
The Quality D atmosphere generally is a background zone, based on how your cleanroom is intended. This is actually the least thoroughly clean area in the GMP necessities for click here sterile items.
Utilize a particle counter that should routinely discover the Higher Assurance Restrict to ascertain exactly where your statistically considerable particle size and variety. Then pick which ISO classification you drop underneath.
The demanded volume of cleanliness for a Grade A area is attained utilizing a laminar stream cupboard or a laminar airflow hood (LAF). In case the bordering air is preserved and classed as Grade B, a laminar flow cabinet can attain a cleanliness classification of Quality A.
It looks like you have been misusing this feature by heading also speedy. You’ve been temporarily blocked from applying it.
Freight Class appreciably influences delivery fees, with LTL carriers using the classification to work out freight costs. Higher classes frequently bring about better rates. Comprehending your freight’s classification is crucial for precise pricing, ensuring that shippers pay back a fair rate for both of those the Place occupied along with the managing demands.
 Amanda Bearse Then & Now!
Amanda Bearse Then & Now! Tiffany Trump Then & Now!
Tiffany Trump Then & Now!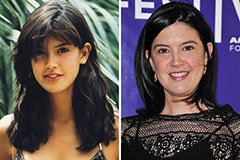 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Lynda Carter Then & Now!
Lynda Carter Then & Now! Katey Sagal Then & Now!
Katey Sagal Then & Now!